Introduction
Atopic dermatitis (AD), a chronic and relapsing inflammatory skin disorder, continues to be a major challenge for both patients and healthcare professionals worldwide. Although AD pathogenesis is complex and not fully understood, multiple exacerbating factors have been identified. The most important triggers include allergic reactions, pollution, sweat, stress, and temperature, to name a few. The question of whether foods can aggravate AD in patients with no food allergies has been a matter of debate for years. Some researchers have proposed that foods rich in histamine, a biogenic amine classically associated with allergic responses, could be a major trigger of AD flare-ups. Nevertheless, no consensus has been reached so far.
This review aims to explore the complex relationship between exogenous histamine and AD, shedding light on its potential contributions to disease flare-ups. We will analyze currently available evidence for the efficacy of implementing a low-histamine diet in AD, with the ultimate goal of advancing our knowledge and providing insights for future therapeutic approaches.
Histamine
Histamine is a biogenic amine and neuro-immuno-endocrine system mediator, found in various tissues of the human body. It is synthesized from the amino acid histidine and plays a crucial role in regulating multiple physiological processes. Histamine functions as a signaling molecule in the central nervous system, where it contributes to various neurological functions, including the sleep-wake cycle and cognitive processes. In the periphery, histamine is released by mast cells and basophils in response to allergens and injury, and it acts as a potent mediator of inflammation and immune responses [1].
Histamine exerts its effects by binding to specific receptors, including H1, H2, H3, and H4, which are located on the surface of target cells. The activation of these receptors leads to a wide range of physiological responses, such as vasodilation, bronchoconstriction, increased gastric acid secretion, and modulation of immune cell activity. Dysregulation of histamine signaling can lead to various pathological conditions, including allergic reactions, gastric ulcers, and autoimmune diseases. As a result, histamine and its receptors are important targets for pharmacological interventions in the treatment of allergies, asthma, acid-related gastrointestinal disorders, and neurological conditions.
The role of histamine in atopic dermatitis
Histamine is a crucial component of the pathophysiology of AD, as well as other atopic diseases, such as allergic rhinitis and asthma. Up to 80% of AD cases in children can be characterized as the extrinsic phenotype, which is driven by skin barrier dysfunction and allergic sensitization with high to extremely high IgE levels [2]. Histamine-mediated mast cell activation leading to a powerful inflammatory cascade is a key mechanism of an IgE-mediated allergic reaction.
What is more, histamine can also enhance the secretion of Th2 cytokines (IL-5, IL-4, IL-10, IL-13) and inhibit Th1 cytokines (IFN-γ, monokine IL-12 and IL-2), leading to Th2 domination [3]. Th2 cytokines (mainly IL4 and IL-13) have been demonstrated to play a key role in the immunological pathways underlying AD pathogenesis [4]. Histamine is also known for its ability to induce itching [5], which remains one of the most burdensome symptoms of AD. Furthermore, histamine can deepen the skin barrier dysfunction via downregulation of the expression of loricrin, filaggrin, keratin 1, and keratin 10 [6].
There is no doubt that endogenous histamine is an important player in AD. The question, however, is whether the exogenous histamine could be of similar importance.
Histamine intolerance
Histamine intolerance (HIT) is a condition characterized by a set of symptoms following ingestion of histamine-rich foods. HIT is a consequence of the accumulation of histamine due to the low activity of the enzyme diamine oxidase (DAO). The causes of this condition are genetic, pathologic (gastro-intestinal diseases, gut microbiome dysbiosis, micronutrient deficiencies), or pharmacologic [1]. HIT should not be mistaken for histamine intoxication (also referred to as histamine poisoning), which is an acute reaction to ingestion of an extreme amount of histamine, often from spoiled or improperly stored foods, such as canned fish [7].
The clinical presentations of HIT are very heterogeneous. One of the organs most commonly affected by HIT is the skin. Patients often experience erythema, especially in the facial area (flushing), intense pruritus, and urticarial rashes. Other manifestations include gastrointestinal symptoms, as well as symptoms related to the nervous, cardiovascular, respiratory, or even reproductive system [1] (Figure 1).
Figure 1
Symptoms of histamine intolerance (HIT). Adapted from Hrubisko M, Danis R, Huorka M, Wawruch M. Histamine intolerance – the more we know, the less we know. A review. Nutrients 2021; 13: 2228. Created in Canva
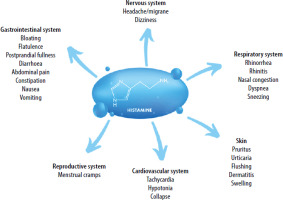
Currently, it is still not clear whether HIT can be diagnosed based on a laboratory assay. The most studied biomarker in HIT patients is the level of the enzyme DAO in serum, using either immunosorbent assay (ELISA) or radioimmunoassay (RIA). Nevertheless, the validity of this diagnostic tool remains controversial. Other potential options include measurement of DAO in the intestinal mucosa, fecal histamine levels, skin prick test, analysis of 1-methylhistamine from urine, and genetic tests (single nucleotide polymorphisms of AOC1 gene). No consensus has been reached so far on whether any of these methods can be used as direct HIT-specific diagnostic criteria [1]. Currently, the most reliable diagnostic option seems to be an in-depth interview with the patient (ideally using a validated scoring system), followed by short-term implementation of the low-histamine diet and tracking of the HIT symptoms. Another possibility is combining the low-histamine diet with a histamine challenge test. However, patients’ reactions to oral provocations were shown to be highly unpredictable and possibly unsafe.
The histamine overlay syndrome
For years HIT has been considered the opposite of allergy – a diagnosis that can be made when allergy is excluded. Many authors refer to this intolerance as a “pseudoallergy”, as it may simulate allergic disease due to the presence of histamine-mediated symptoms. Currently, more and more evidence is pointing to the so-called overlay syndrome, within which the ingestion of exogenous histamine can worsen the course of allergic and atopic diseases due to a degree of histamine overload [8]. Not all patients experiencing worsening of skin lesions and pruritus after ingestion of histamine-rich foods can be diagnosed with HIT. In AD patients, exogenous histamine can only lead to symptoms during intensification of allergic reactions (e.g. in the spring due to contact with pollen). Amounts of ingested histamine leading to symptoms may also be highly varied. Perhaps, in a subgroup of histamine-sensitive atopic patients, we should refer to this condition as the histamine overlay syndrome rather than intolerance.
Histamine metabolism in AD
Exogenous histamine is metabolized via two main enzymatic pathways: by the enzymes diamine oxidase (DAO) and histamine N-methyltransferase (HNMT). The DAO pathway is considered to be the most clinically significant as it is responsible for the great majority of dietary histamine metabolism. In the human body, DAO can be found on epithelial cells of the small intestine, the placenta, and the kidneys, as well as the thymus and seminal plasma. Continuous synthesis of DAO in the intestine during eating is necessary for the proper metabolism of ingested histamine and regulation of inflammation, proliferation, allergic responses, and ischemia [1]. HNMT is a cytosolic enzyme, which can also be found in the gastrointestinal tract, as well as in numerous other tissues (e.g. brain, bronchi, liver, kidneys, ovary, spinal cord). Nevertheless, it is thought to have a much smaller role in the metabolism of ingested histamine [9]. However, HNMT is crucial for the metabolism of endogenous histamine, for example in the central nervous system [10].
It has been suggested that the activity of the enzyme DAO may be reduced in patients with AD, leading to worsening of histamine-mediated reactions. In a 2006 study, levels of the plasma histamine and the DAO were measured in 3 groups of participants: patients with AD (n = 162), patients with histamine intolerance, but no AD symptoms (n = 124), and healthy controls (n = 85). Symptoms of histamine intolerance were evaluated in all the groups. Interestingly, DAO enzyme activity (measured with ELISA) was significantly lower in patients with AD than healthy controls (p < 0.001) (Table 1). At the same time, histamine plasma levels were significantly higher in patients with AD than healthy controls (p < 0.05). In patients with AD sensitization towards food allergens (hazelnut, peanut, milk, egg, apple, and codfish) and occurrence of histamine-related symptoms (headache, gastrointestinal symptoms) was observed in a significantly higher number of patients with AD and low DAO compared with those with AD but a normal DAO level. This observation suggests a potential role of DAO deficiency in exacerbating allergic reactions in AD [11].
Table 1
Low and normal diamine oxidase (DAO) activity in serum from patients with suspected histamine intolerance (HIT), patients with atopic dermatitis (AD), and a healthy control group (CTR). Adapted from Maintz L, et al. Evidence for a reduced histamine degradation capacity in a subgroup of patients with atopic eczema. Journal of Allergy and Clinical Immunology 2006; 117: 1106-12. https://doi.org/10.1016/j.jaci.2005.11.041.
| Parameter | Total number | Low DAO | Normal DAO | P-value | χ2 | ||
|---|---|---|---|---|---|---|---|
| n | n | % | n | % | |||
| CTR | 85 | 0 | 0 | 85 | 100 | ||
| AD | 162 | 31 | 19.14 | 131 | 80.86 | < 0.001 | 18.6 |
| HIT | 124 | 25 | 20.16 | 99 | 79.84 | < 0.001 | 19.466 |
Very similar results were obtained in a 2019 study, which analyzed the ability to degrade histamine in 53 patients with suspected allergies. All participants were skin prick tested with histamine and the resolution of the wheal was monitored for 50 min. Blood DAO activity and histamine concentration were measured with a radio-extraction radioimmunoassay. Interestingly, allergic patients were characterized by significantly lower DAO activity and higher histamine levels compared to healthy subjects. Moreover, in patients with slow resolution of the histamine-induced wheal a clear correlation with high histamine levels and low DAO activity was observed [12]. Although AD is not an allergy, the results of this study are of great significance. As many as 80% of AD cases are of the extrinsic type, characterized by high IgE levels and the coexistence of allergies [13].
Furthermore, in a 2008 study, reduced enzymatic inactivation via HNMT was observed in AD patients. The authors suggested that increased histamine levels and symptoms related to it may result, at least in part, from reduced enzymatic inactivation via HNMT due to genetic predisposition [14]. Although HNMT activity does not seem to play a significant role in the metabolism of ingested histamine in the gut, it has an important role in the metabolism in the bronchi and the skin. Therefore, the results of both studies suggest that the limited ability to metabolize histamine may be a hallmark of AD, at least in a subpopulation of patients.
Finally, when compared to control subjects, patients with severe AD have shown higher basal plasma histamine concentrations and increased spontaneous histamine release in response to multiple stimuli, and after food challenges [15, 16]. It is worth pointing out that these data come from two rather outdated studies. Nevertheless, currently available data points towards limited histamine metabolism in AD, possibly leading to histamine overlay syndrome after ingestion of histamine-rich foods.
The low-histamine diet in AD
So far, the low-histamine diet in AD has been investigated only in a single study, conducted in 2009. The intervention consisted of the implementation of a histamine-free diet for 2 weeks in patients with AD (n = 36). Additionally, sources of other biogenic amines (preservatives, coloring agents, antioxidants, salicylates, benzoates, and aromatic compounds) were also eliminated. During the second week of the diet, oral histamine double-blind placebo-controlled provocations were performed in AD patients and healthy controls (n = 19). Blood samples for measurement of serum histamine and DAO were collected at the start of the study, before and 30 min after each histamine challenge from both groups [17].
Plasma histamine was significantly higher in patients with AD compared with controls (p < 0.001), whereas DAO activity was similar in both groups. Eleven out of 36 patients showed exacerbation of AD symptoms after histamine oral provocations. Twelve of 36 patients showed a significant improvement of the SCORAD after 1 week of the diet. Interestingly, after the high dose histamine provocation (1.5 mg/kg of body weight) 8 patients with AD had to stop the study because of hypotension. By contrast, within the control group only mild systemic reactions such as flush occurred, which did not require medical intervention. Surprisingly, patients with AD and severe reactions to histamine provocations did not have low DAO levels. This observation shows that DAO serum measurement may not be a valid diagnostic tool, at least in patients with suspected sensitivity to exogenous histamine.
Overall, the results of the study suggest that sensitivity to exogenous histamine may be higher in AD patients when compared to healthy controls. Also, the low-histamine diet may lead to an improvement of AD symptoms in around 30% of patients. We cannot draw clear conclusions from a single study (especially considering the small study population and no control diet). Nevertheless, this topic should be further investigated.
In 2011 a case report of successful treatment of AD with a low-histamine diet was published. The report describes a 6-year-old boy who had suffered from itchy, erythematous patches on his face and extremities since he was 3 months old. The treatment with topical steroids, antihistamines, and cyclosporine did not lead to any improvement. It was suspected that the boy suffered from an allergy to pork. Interestingly, the oral challenge showed reactions to 200 g of pork, but not to 60 g of pork, which is highly unusual in patients with allergies, but quite common in patients with HIT. The analysis of the patient’s diet revealed that histamine-rich foods were consumed often and in high amounts (e.g. soy sauce, kimchi, mackerel, spinach). The patient’s parents were instructed to implement a balanced, low-histamine diet. After 1 month of the diet, the administration of topical immunomodulators (Protopic) and oral histamines was no longer needed. After 3 months of the diet, the boy only had slight erythematous patches on flexural areas of limbs and neck without any pruritus. His EASI and VAS scores went down from 15.6 and 1.2 to 0.3 and 0, respectively. After 7 months no signs or symptoms of AD could be observed [18].
Similar conclusions come from a review paper published in 2022 in the Polish Journal of Allergology by K. Buczyłko. The review is based on the author’s observations from clinical practice. He described multiple cases of his patients who had experienced exacerbation of atopy and/or allergy due to ingestion of histamine-rich foods. Supplementation of DAO and a low-histamine diet led to a significant improvement in symptoms [8].
The role of the gut microbiome
Numerous studies have shown that AD is closely linked to alterations in bacterial diversity and composition in the gut microbiome [19]. Moreover, patients with AD experience gastrointestinal disorders more often than the healthy population [20]. It is well established that gut dysbiosis is one of the main drivers of DAO deficiency and HIT [21]. Interestingly, certain strains of bacteria in the human microbiome can synthesize histamine, leading to higher histamine blood levels [22]. Therefore, it could be hypothesized that in patients experiencing AD exacerbation after ingestion of histamine-rich foods the limited ability to metabolize histamine derives not only from genetic predisposition but also from dysfunction in gut microbiome composition, leading to increased histamine production and decreased DAO synthesis. Currently, there is no evidence for increased microbial production of histamine in AD. However, this hypothesis could be the focus of fascinating future research.
Future perspectives
Currently available evidence points to: 1) increased histamine levels in AD; 2) decreased histamine metabolism in AD; 3) therapeutic potential of the low-histamine diet in a subgroup of histamine-sensitive AD patients. Future research should focus on establishing the prevalence of reactions related to ingestion of exogenous histamine in AD. Moreover, reliable screening tools should be created – possibly a detailed, validated questionnaire with a scoring system. Finally, the influence of the low-histamine diet in histamine-sensitive AD patients should be established.







